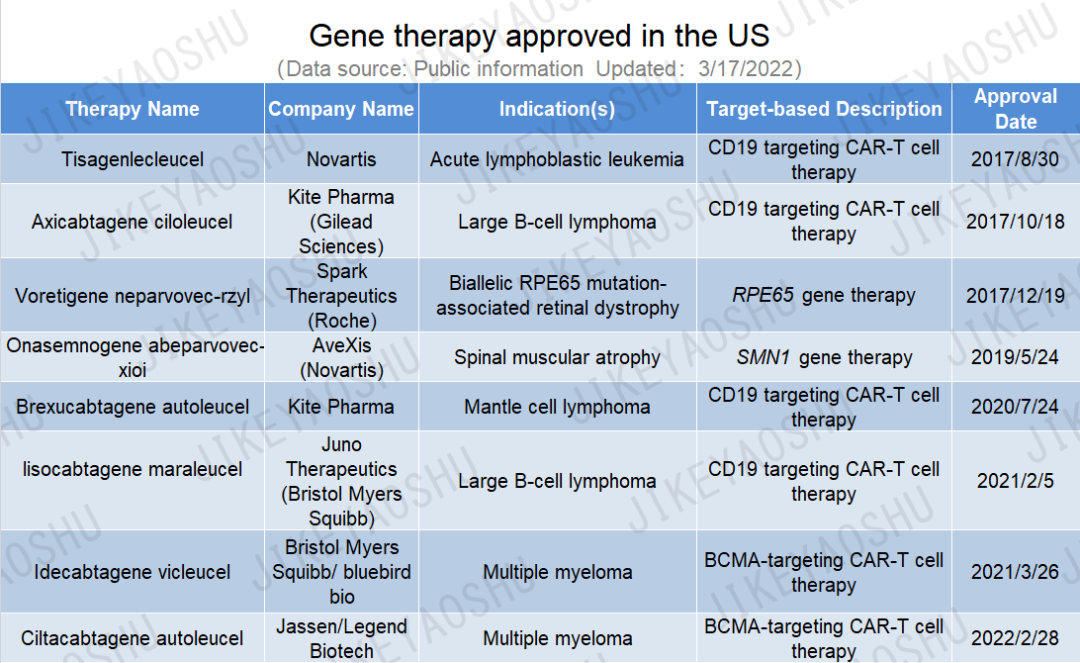
自2017年8月(Kymriah)tisagenlecleucel在美国首次批准用于治疗急性淋巴细胞白血病以来,目前至少已有8个基因疗法获美国FDA批准上市,其中包括最近批准的Carvykti(ciltacabtagene autoleucel),详见下表。

(药明康德内容团队制图,点击可见大图,括号中公司收购了产品的研发公司)
在刚刚过去的2月份,已有杨森(Janssen)和传奇生物联合开发的Carvykti获得FDA批准,今年还有3种基因疗法可能获得FDA批准,另外8种预计在年底前提交生物制品许可申请(BLA)。
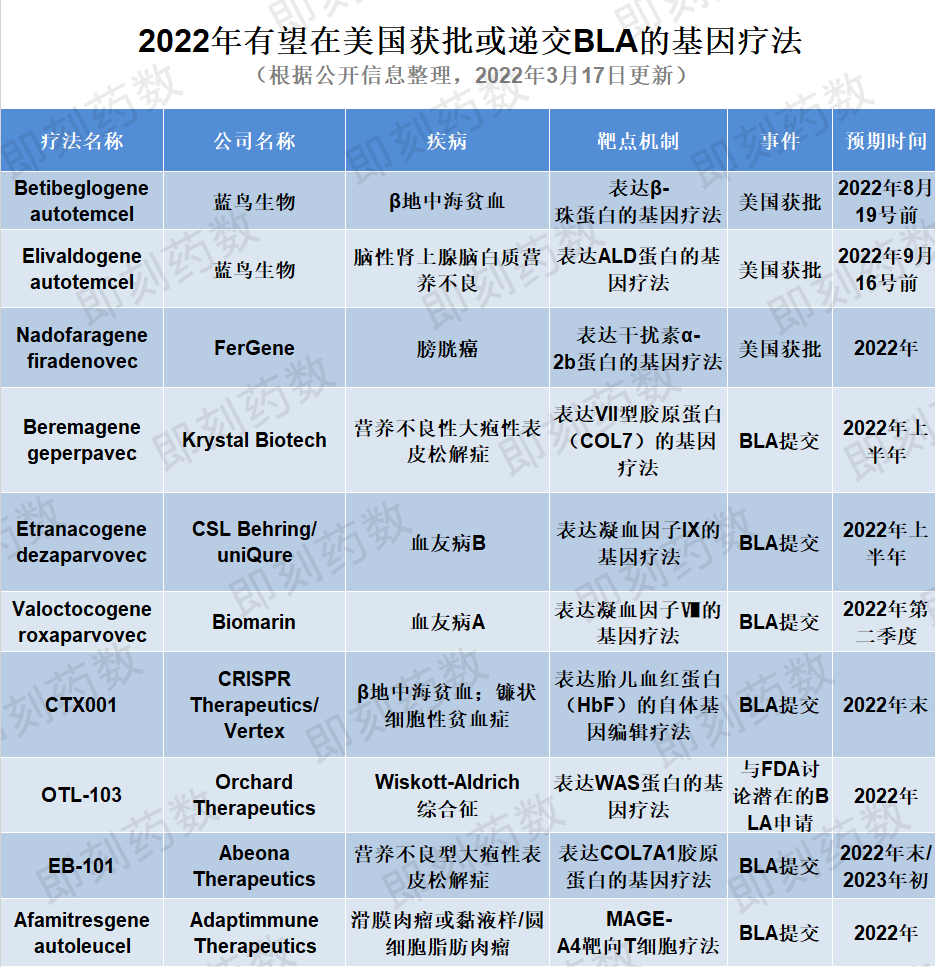
(药明康德内容团队制图,点击可见大图)
1. 疗法名称:Betibeglogene autotemcel
公司名称:蓝鸟生物
适应症:β地中海贫血
2022年蓝鸟生物公司有两种基因疗法预计将获得FDA批准。Betibeglogene autotemcel(beti-cel)是一种造血干细胞体外基因疗法,用于治疗需要接受常规血红细胞输注的β地中海贫血患者。该疗法从患者体内分离出造血干细胞,并利用病毒载体引入经过修饰、能行使正常功能的β球蛋白基因,是一种潜在的一次性治疗基因疗法。目前该BLA申请正在接受FDA审评,PDUFA目标日期设定为2022年8月19日。蓝鸟生物预计FDA外部专家委员会将在2022年6月召开会议讨论这一申请。目前该疗法已获FDA优先审评资格,如果获得FDA的批准,预计该疗法会成为潜在的美国首个针对β地中海贫血患者的慢病毒载体基因疗法。
2. 疗法名称:Elivaldogene autotemcel
公司:蓝鸟生物
适应症:脑肾上腺脑白质营养不良
FDA正在审评的蓝鸟生物的第二种基因疗法是elivaldogene autotemcel(eli-cel),治疗18岁以下携带ABCD1基因突变的早期脑性肾上腺脑白质营养不良患者(CALD)。这种疾病是由于ABCD1基因突变引起的,这种突变影响了ALD蛋白的产生,随后导致极长链脂肪酸(VLCFAs)的毒性积累,主要发生在肾上腺、大脑与脊髓的白质中。FDA正在审评该BLA申请,PDUFA目标日期为2022年9月16日。与beti-cel一样,蓝鸟生物预计FDA外部专家委员会将在2022年6月召开会议。
3. 疗法名称:Nadofaragene firadenovec
公司名称:FerGene(Ferring)
适应症:膀胱癌
2022年FDA正在审查的基因疗法还包括nadofaragene firadenovec(Instiladrin),由Ferring Pharmaceuticals的子公司FerGene开发,用于治疗对卡介苗(BCG)响应不佳的晚期高级非肌层浸润性膀胱癌(high grade NMIBC)。Nadofaragene firadenovec最初由芬兰基因疗法公司FKD Therapies开发,Ferring在2018年与FKD Therapies达成合作共同开发这一基因疗法,并且将这一资产转给了FerGene公司。这种全新的基因疗法机制可以将患者自身膀胱壁细胞转化为制造多种干扰素的微型工厂,增强机体抵抗癌症的天然防御能力。2019年,积极的3期结果显示该疗法已达到其主要疗效终点,并且具有良好的安全性和耐受性。此前FDA曾授予其快速通道资格,突破性疗法认定和优先审评资格,并接收递交的BLA。如果2022年获得批准,该疗法将为对BCG无反应的NMIBC患者提供一个有希望的选择。
4. 疗法名称:Beremagene geperpavec
公司:Krystal Biotech
适应症:营养不良性大疱性表皮松解症
对于即将在美国提交BLA的基因治疗候选药物,第一个介绍的是Krystal Biotech的beremagene geperpavec,一种表达VII型胶原蛋白(COL7)的局部基因疗法,用于治疗营养不良性大疱性表皮松解症(DEB),这是一种罕见且严重的疾病,影响皮肤和黏膜组织。该疗法旨在通过为患者的皮肤细胞提供制造正常COL7蛋白的模板,在分子水平上治疗DEB。继2021年11月公布的积极安全性和有效性3期结果后,该公司有望在2022年上半年向美国FDA提交BLA。
5. 疗法名称:Etranacogene dezaparvovec
公司:uniQure、CSL Behring
适应症:血友病B
Etranacogene dezaparvovec由uniQure与CSL Behring合作开发,旨在使用腺相关病毒5(AAV5)载体递送表达FIX Padua变体的基因,用于治疗中重度至重度血友病B。已在其3期试验中证明了安全性并达到了主要和次要疗效终点。CSL计划在2022年上半年在美国和欧盟提交该疗法的上市申请。如果获得批准,etranacogene dezaparvovec可能是潜在的第一个为血友病B患者提供持久、功能性治疗益处的基因疗法。
6. 疗法名称:Valoctocogene roxaparvovec
公司名称:BioMarin Pharmaceutical
适应症:血友病A
BioMarin的基因疗法候选药物valoctocogene roxaparvovec也是一种基于AAV5的基因疗法,准备在今年提交BLA。该疗法用AAV5病毒载体递送表达凝血因子VIII的转基因,用于治疗血友病A,这是一种遗传性罕见的出血性疾病。BioMarin最初于2019年12月向FDA提交了BLA,FDA要求提供进一步的后续数据。2022年1月,公司公布了其全球性3期临床试验的两年随访安全性和有效性数据,显示主要和次要终点均已达到。BioMarin计划于2022年第二季度在美国重新提交其BLA,用于治疗严重的血友病A,预计最早获批时间为2022年底。
7. 疗法名称:Eladocagene exuparvovec
公司:PTC Therapeutics
适应症:芳香族 L-氨基酸脱羧酶缺乏症
PTC Therapeutics的eladocagene exuparvovec(PTC-AADC)是一种基因替代疗法,单剂量将人类多巴脱羧酶(DDC)基因递送到大脑的壳核中,用于治疗芳香族L-氨基酸脱羧酶缺乏症(AADCD),一种影响大脑的罕见遗传疾病。来自三个1期和2期临床试验的5年结果分析表明,AADCD儿童的运动功能和认知能力持续得到改善。公司计划在2022年第二季度提交BLA。如果获得批准,这将是AADCD患者潜在的第一个上市治疗方式。目前,根据公开领域信息,在该疾病领域尚未有其他基因疗法处于后期临床试验中。
8. 疗法名称:Afamitresgene autoleucel
公司:Adaptimmune Therapeutics
适应症:膜肉瘤或黏液样/圆细胞脂肪肉瘤(MRCLS)
Afamitresgene autoleucel(afami-cel)是Adaptimmune Therapeutics公司的开发的工程化靶向MAGE-A4抗原的T细胞疗法。该疗法在其正在进行的滑膜肉瘤和粘液样/圆细胞脂肪肉瘤(MRCLS)2期研究中达到了主要疗效终点。基于积极的结果,Adaptimmune准备在2022年第四季度提交一份用于滑膜肉瘤的BLA申请。目前,手术是滑膜肉瘤的首选治疗方法,有些病人会在手术前后接受放射治疗。如果获得批准,afami-cel将为该患者群体提供具有更高生活质量的替代治疗。
9. 疗法名称:CTX001
公司名称:CRISPR Therapeutics、Vertex Pharmaceuticals
适应症:β地中海贫血;镰刀型细胞贫血病
CTX001是一种非病毒载体的基因编辑疗法,由CRISPR Therapeutics和Vertex合作开发,用于治疗患有输血依赖性β地中海贫血(TDT)或严重镰刀型细胞贫血病(SCD)的患者,通过在体外对患者的造血干细胞进行改造,使红细胞中产生高水平的胎儿血红蛋白(HbF)。该疗法已完成其正在进行的3期研究的患者注册,并已对70多名患者进行了给药。CTX001已获得美国FDA授予治疗TDT和SCD的再生医学先进疗法(RMAT)认定、以及快速通道和孤儿药资格。Vertex计划在2022年底提交CTX001用于治疗这两种适应症的全球(包括美国FDA)监管申请文件。
10. 疗法名称:EB-101
公司:Abeona Therapeutics
适应症:隐性营养不良型大疱性表皮松解症
今年有望在美国递交上市申请的另外一种COL7A1基因靶向疗法是Abeona的EB-101。该疗法是一种体外基因校正的自体细胞疗法,将COL7A1胶原蛋白基因插入到患者自身的皮肤细胞(角质形成细胞)中。该疗法正在一项3期安全性和有效性临床试验中进行研究,用于治疗隐性营养不良型大疱性表皮松解症(RDEB),这是一种罕见的结缔组织疾病,目前还没有获得批准的疗法。在1/2期研究中,EB-101已显示出可观的伤口愈合和长达六年的长期疼痛减轻效果。Abeona已完成其3期试验的患者注册,并预计将在2022年第三季度提供一线结果,随后将在2022年底或2023年初提交BLA申请。
11. 疗法名称:OTL-103
公司名称:Orchard Therapeutics
适应症:Wiskott-Aldrich综合征
Orchard Therapeutics的OTL-103是一种自体造血干细胞基因疗法,包括用编码Wiskott-Aldrich综合征(WAS)基因的慢病毒载体体外转染的CD34+细胞,旨在通过单次注射治疗Wiskott-Aldrich综合征,这是一种罕见的遗传性免疫缺陷紊乱。该疗法已获得FDA授予的孤儿药资格和罕见儿科疾病(RPD)资格。目前,评估疗效和安全性的3期临床试验正在进行中,Orchard计划在2022年初与FDA讨论其潜在的BLA申请。
2022 FDA Regulatory Outlook for Gene Therapy
Since the first gene therapy approval of tisagenlecleucel (Kymriah) in the US for the treatment of acute lymphoblastic leukemia in August 2017, there have been 8 more FDA approvals for gene therapies, including voretigene neparvovec-rzyl (Luxturna), an adeno-associated virus vector-based gene therapy for the treatment of confirmed biallelic RPE65 mutation-associated retinal dystrophy, as well as the most recently approved ciltacabtagene autoleucel (Carvykti) , a cell-based gene therapy for the treatment of relapsed or refractory multiple myeloma.
(By WuXi AppTec content team. Click the image to view at full size, the company in () acquired the company developing the product)
Besides ciltacabtagene autoleucel co-developed by Jassen and Legend Biotech, which was approved last month by the FDA, there are 3 additional gene therapies which could gain FDA approval and another 8 aiming for BLA submissions by the end of the year.
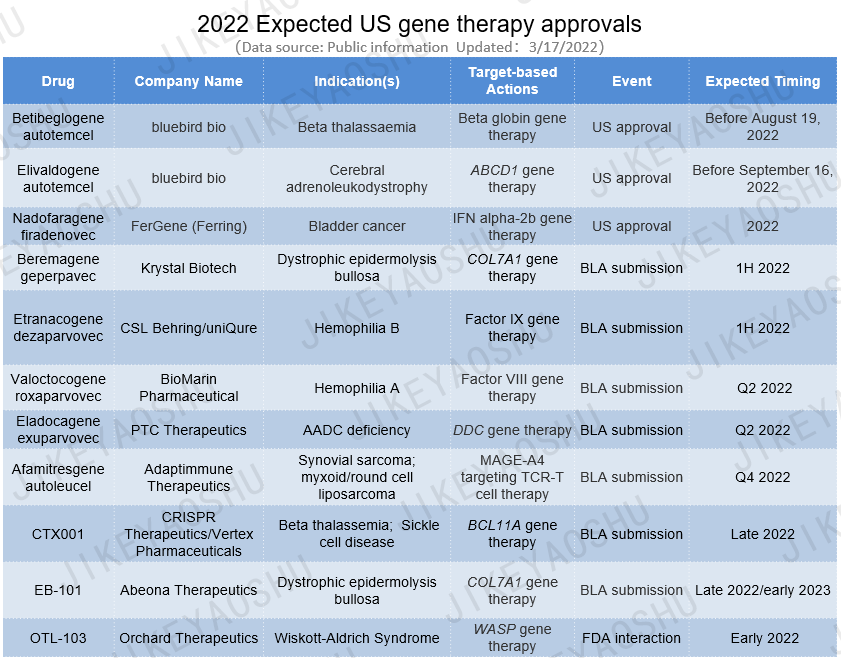
(By WuXi AppTec content team. Click the image to view at full size)
1. Drug: Betibeglogene autotemcel
Companies: bluebird bio
Indication(s): Beta thalassemia
One of the major gene therapy developers to watch in 2022 is bluebird bio. The company has two gene therapies which are expecting FDA approval this year. Betibeglogene autotemcel (beti-cel) is a cell-based gene therapy comprising autologous CD34+ hematopoietic stem cells (HSCs) transduced ex vivo with lentiglobin BB305 lentiviral vector encoding the beta-A-T87Q-globin gene, designed to enable normal red blood cell formation in patients and treat the underlying cause of beta thalassaemia. The BLA filing for beti-cel for the treatment of beta thalassaemia in patients who receive regular red blood cell transfusions is currently under review by the FDA, with the PDUFA date goal date set as August 19, 2022. bluebird is expecting an FDA advisory committee meeting in June 2022 before the approval decision. If the product is approved by the FDA under the current schedule, it could potentially be the first lentiviral vector gene therapy for patients with beta thalassaemia in the US.
2. Drug: Elivaldogene autotemcel
Company: bluebird bio
Indication(s): Cerebral adrenoleukodystrophy
The second gene therapy from bluebird under review by the FDA is elivaldogene autotemcel (eli-cel), a cell-based gene therapy product in which hematopoietic stem cells from the patient are transduced ex vivo with the Lenti-D lentiviral vector to add functional copies of the ABCD1 gene, for the treatment of cerebral adrenoleukodystrophy, a severe rare X-linked metabolic disorder. If untreated, it can lead to severe loss of neurological function and eventual death in most patients. The FDA is reviewing the BLA for eli-cel for the treatment of cerebral adrenoleukodystrophy in patients under 18 years of age, with the PDUFA goal date of September 16, 2022. Same as beti-cel, bluebird is anticipating an FDA advisory committee meeting for eli-cel in June 2022 before the approval decision.
3. Drug: Nadofaragene firadenovec
Company: FerGene (Ferring)
Indication(s): Bladder cancer
The third gene therapy under review by the FDA is nadofaragene firadenovec (Instiladrin), being developed by FerGene, a subsidiary of Ferring, for the treatment of high grade, bacillus Calmette-Guerin (BCG) unresponsive non-muscle invasive bladder cancer (NMIBC). The therapy was previously being developed by FKD Therapies under exclusive license from MSD, and FerGene has later obtained global commercialization rights to the therapy. Nadofaragene firadenovec comprises a recombinant adenovirus vector harboring the human interferon alpha 2b gene, administered once every 3 months intravesically in combination with excipient Syn3 to enhance transduction of the virus into the epithelial cell lining in the bladder. A BLA filing has been submitted by FKD for nadofaragene firadenovec based on positive phase III results demonstrating that the therapy has met its primary efficacy endpoint, as well as having favorable safety and tolerability. The current recommended treatment for high-grade NMIBC is intravesical BCG, with a recurrence rate of 30% to 50% and total cystectomy often being the next treatment option. Ferring’s pipeline indicated that the BLA is with the FDA and approval could still happen this year. If approved, the therapy would provide a promising option for NMIBC patients who are unresponsive to BCG.
4. Drug: Beremagene geperpavec
Company: Krystal Biotech
Indication(s): Dystrophic epidermolysis bullosa
For the upcoming gene therapy candidates getting ready for BLA submission in the US, the first on the list is Krystal Biotech’s beremagene geperpavec, a topical COL7A1-expressing gene therapy for the treatment of dystrophic epidermolysis bullosa (DEB), a rare and severe disease that affects the skin and mucosal tissues. The therapy is aimed to address the disease-causing mechanism by providing the patient’s skin cells the template to make normal COL7 protein. Following positive safety and efficacy phase III results published in November 2021, the company is on track for BLA submission with the US FDA in 1H 2022.
5. Drug: Etranacogene dezaparvovec
Companies: CSL Behring, uniQure
Indication(s): Hemophilia B
Etranacogene dezaparvovec is an adeno-associated virus 5 (AAV5)-based gene therapy carrying a gene cassette with the Padua variant of Factor IX, being developed by uniQure in collaboration with CSL Behring as a treatment for severe and moderately severe hemophilia B. The product has demonstrated safety and met both primary and secondary efficacy endpoints in its phase III trial. CSL is planning to submit marketing application for the therapy both in the US and EU in 1H 2022. If approved, etranacogene dezaparvovec could be the first gene therapy to provide durable, functionally curative benefits to hemophilia B patients, with optimized clinical and safety benefits.
6. Drug: Valoctocogene roxaparvovec
Company: BioMarin Pharmaceutical
Indication(s): Hemophilia A
BioMarin’s gene therapy candidate, valoctocogene roxaparvovec, is also an AAV5-based gene therapy preparing for BLA filing this year. The therapy contains a B-domain-deleted FVIII gene and a liver-specific promoter, for treatment of hemophilia A, an inherited rare bleeding disorder. BioMarin originally filed a BLA to the FDA in December 2019 for which the FDA requested further follow-up data. In January 2022, the company announced favorable two-year follow-up safety and efficacy data from its global phase III trial, showing that both primary and secondary endpoints were met. The results suggested the potential of the therapy for sustained hemostatic bleed control for hemophilia A. Based on the follow-up data obtained, BioMarin intends to resubmit its BLA in the US in Q2 2022 for the treatment of severe hemophilia A, with expected approval as early as the end of2022.
7. Drug: Eladocagene exuparvovec
Companies: PTC Therapeutics
Indication(s): Aromatic L-amino acid decarboxylase deficiency
PTC’s eladocagene exuparvovec (PTC-AADC) is an investigational gene replacement therapy, designed as a single dose to deliver the human dopa decarboxylase (DDC) gene into the putamen, for the treatment of aromatic L-amino acid decarboxylase (AADC) deficiency, a rare genetic disorder that affects the brain. Analysis from 5-year results from three phase I and II clinical trials have shown sustained motor function improvements, cognitive skills in children with AADC deficiency. The company plans to submit a BLA in Q2 2022. If approved, it will be the first commercial treatment for AADC deficiency patients. Currently, there are no other gene therapies in late stage clinical trials for the disorder.
8. Drug: Afamitresgene autoleucel
Companies: Adaptimmune Therapeutics
Indication(s): Synovial sarcoma; myxoid/round cell liposarcoma
Afamitresgene autoleucel (afami-cel) is Adaptimmune’s first-generation engineered TCR-T cell therapy comprising autologous genetically modified T cells for the treatment of MAGE-A4 positive tumors. The therapy has met primary efficacy endpoint in its ongoing phase II study for synovial sarcoma and myxoid/round cell liposarcoma, with encouraging durability. Based on the positive results, Adaptimmune is preparing to submit a BLA filing for afami-cel for synovial sarcoma in Q4 2022. Currently, surgery is the first choice of treatment for synovial sarcomas, sometimes with radiation therapy before/after the surgery. If approved, afami-cel would offer an alternative treatment with a higher quality of life for this patient group.
9. Drug: CTX001
Companies: CRISPR Therapeutics, Vertex Pharmaceuticals
Indication(s): Beta thalassemia; Sickle cell disease
CTX001 is a non-viral gene-editing therapy comprising autologous CD34+ hematopoietic stem and progenitor cells modified ex vivo with CRISPR-Cas9 at the erythroid lineage-specific enhancer of the BCL11A gene, being developed by CRISPR Therapeutics and Vertex, as a potential functional cure for transfusion-dependent thalassemia (TDT) and severe sickle cell disease (SCD). The therapy has completed enrollment in its ongoing phase III studies and have dosed more than 70 patients. It is potentially the most advanced gene-editing approach in development for TDT and SCD. Vertex is planning to submit global regulatory filings for CTX001 in both indications in late 2022.
10. Drug: EB-101
Companies: Abeona Therapeutics
Indication(s): Dystrophic epidermolysis bullosa
Another COL7A1 gene-targeting therapy ready for marketing submission in the US this year is Abeona’s EB-101. This therapy comprises autologous, gene-corrected keratinocyte sheets and is being investigated in a phase III safety and efficacy study for the treatment of recessive dystrophic epidermolysis bullosa, a rare connective tissue disorder without an approved therapy. EB-101 has shown considerable wound healing and reduction in associated long-term pain for up to six years in a phase I/IIa study. Abeona has achieved target enrollment its phase III trial and expects topline results to be available in Q3 2022, followed by BLA filing, expected in late 2022 or early 2023.
11. Drug: OTL-103
Companies: Orchard Therapeutics
The final gene therapy candidate which is in preparation to go through the FDA regulatory submission process is Orchard’s OTL-103. The autologous hematopoietic stem cell gene therapy comprises CD34+ cells transduced ex-vivo with a lentiviral vector encoding Wiskott-Aldrich syndrome (WAS) gene, and is designed to be administered through a single injection to treat Wiskott-Aldrich syndrome, a rare inherited immune deficiency disorder. In the US, the therapy has received Orphan drug designation and a rare pediatric disease designation for the treatment of WAS. At the time of writing, phase III registration trial to assess efficacy and safety is ongoing and Orchard is planning to interact with the FDA in early 2022 to discuss its potential BLA filing package.
参考资料:
[1]FDA approval brings first gene therapy to the United States. Retrieved on March17, 2022 from, https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states
[2]Approved Cellular and Gene Therapy Products. Retrieved on March 17, 2022 from, https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-[]cellular-and-gene-therapy-products
[3]FDA Approves Spark Therapeutics’ LUXTURNA™ (voretigene neparvovec-rzyl), aOne-time Gene Therapy for Patients with Confirmed Biallelic RPE65Mutation-associated Retinal Dystrophy. Retrieved on March 17, 2022 from, https://sparktx.com/press_releases/fda-approves-spark-therapeutics-luxturna-voretigene-neparvovec-rzyl-a-one-time-gene-therapy-for-patients-with-confirmed-biallelic-rpe65-mutation-associated-retinal-dystrophy/
[4]U.S. FDA Approves CARVYKTI™ (ciltacabtagene autoleucel), Janssen’s First CellTherapy, a BCMA-Directed CAR-T Immunotherapy for the Treatment of Patients withRelapsed or Refractory Multiple Myeloma. Retrieved on March 17, 2022 from, https://www.jnj.com/u-s-fda-approves-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-a-bcma-directed-car-t-immunotherapy-for-the-treatment-of-patients-with-relapsed-or-refractory-multiple-myeloma
[5]Zynteglo | European Medicines Agency. Retrieved on March 17, 2022 from, https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo#authorisation-details-section
[6]bluebird bio Reports Fourth Quarter and Full Year 2021 Financial Results,Highlights Operational Progress and Provides Corporate Update. Retrieved onMarch 17, 2022 from, https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-reports-fourth-quarter-and-full-year-2021-financial
[7]bluebird Provides Update on FDA Review Timelines for Betibeglogene Autotemcel(beti-cel) for Beta-Thalassemia and Elivaldogene Autotemcel (eli-cel) for CerebralAdrenoleukodystrophy (CALD). Retrieved on March 17, 2022 from, https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-provides-update-fda-review-timelines-betibeglogene
[8]FKD Therapies Acquires Exclusive License to Merck & Co.’s Gene TherapyPortfolio. Retrieved on March 17, 2022 from, https://www.genengnews.com/topics/genome-editing/fkd-therapies-acquires-exclusive-license-to-merck-co-s-gene-therapy-portfolio/
[9]Ferring signs global agreement to commercialise novel gene therapy for bladdercancer patients. Retrieved on March 17, 2022 from, https://ferringusa.com/?press=ferring-signs-global-agreement-to-commercialise-novel-gene-therapy-for-bladder-cancer-patients
[10]INSTILADRIN® in Patients With Bacillus Calmette-Guerin (BCG) UnresponsiveNon-Muscle Invasive Bladder Cancer (NMIBC). Retrieved on March 17, 2022 from, https://clinicaltrials.gov/ct2/show/NCT02773849
[11]Ferring and Blackstone Life Sciences invest over $570 million USD in novel genetherapy for bladder cancer patients. Retrieved on March 17, 2022 from, https://ferringusa.com/?press=ferring-and-blackstone-life-sciences-invest-over-570-million-usd-in-novel-gene-therapy-for-bladder-cancer-patients
[12]Krystal Biotech Announces Positive Topline Results from GEM-3 Pivotal Trialof VYJUVEK™ in Patients with Dystrophic Epidermolysis Bullosa. Retrievedon March 17, 2022 from, https://ir.krystalbio.com/news-releases/news-release-details/krystal-biotech-announces-positive-topline-results-gem-3-pivotal
[13]uniQure Announces 2021 Financial Results and Highlights Recent CompanyProgress. Retrieved on March 17, 2022 from, https://tools.eurolandir.com/tools/Pressreleases/GetPressRelease/?ID=4046783&lang=en-GB&companycode=nl-qure&v=
[14]Induction of ER Stress by an AAV5 BDD FVIII Construct Is Dependent on theStrength of the Hepatic-Specific Promoter. Retrieved on March 17, 2022 from, https://pubmed.ncbi.nlm.nih.gov/32775496/
[15]BioMarin Announces Fourth Quarter and Full Year 2021 Financial Results andCorporate Updates. Retrieved on March 17, 2022 from, https://investors.biomarin.com/2022-02-23-BioMarin-Announces-Fourth-Quarter-and-Full-Year-2021-Financial-Results-and-Corporate-Updates
[16]BioMarin Announces Stable and Durable Annualized Bleed Control in the LargestPhase 3 Gene Therapy Study in Adults with Severe Hemophilia A; 134-ParticipantStudy Met All Primary and Secondary Efficacy Endpoints at Two Year Analysis.Retrieved on March 17, 2022 from, https://investors.biomarin.com/2022-01-09-BioMarin-Announces-Stable-and-Durable-Annualized-Bleed-Control-in-the-Largest-Phase-3-Gene-Therapy-Study-in-Adults-with-Severe-Hemophilia-A-134-Participant-Study-Met-All-Primary-and-Secondary-Efficacy-Endpoints-at-Two-Year-Analysis
[17]A Safety and Efficacy Study Evaluating CTX001 in Subjects With Severe SickleCell Disease. Retrieved on March 17, 2022 from, https://clinicaltrials.gov/ct2/show/NCT03745287?term=CTX001&draw=2&rank=2
[18]Vertex Reports Fourth Quarter 2021 and Full Year Financial Results. Retrieved on March 17, 2022 from, https://news.vrtx.com/press-release/vertex-reports-fourth-quarter-2021-and-full-year-financial-results
[19]A Clinical Study to Evaluate the Use of a Cryopreserved Formulation of OTL-103in Subjects With Wiskott-Aldrich Syndrome. Retrieved on March 17, 2022 from, https://clinicaltrials.gov/ct2/show/NCT03837483?term=OTL-103&draw=2&rank=1
[20]40th Annual JP Morgan Healthcare Conference – Orchard Therapeutics. Retrievedon March 17, 2022 from, https://ir.orchard-tx.com/static-files/49828890-db79-452f-a580-845533886cb7
[21]Orchard Therapeutics Annual Report and Accounts for the Year Ended 31 December2020. Retrieved on March 17, 2022 from, https://ir.orchard-tx.com/static-files/dc78e950-dd1d-4934-8f4d-6725e9bf4a6f
关于举办2026年度四川省药品生产企业质
各药品生产企业: 2026年是我国..四川省医药保化品质量管理协会召开第七
2025年12月17日,四川省医药保化品..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..