近日,美国FDA发布了2021财年检查观察项的汇总数据,对本财年483表格中引用的相关缺陷数据进行了总结。
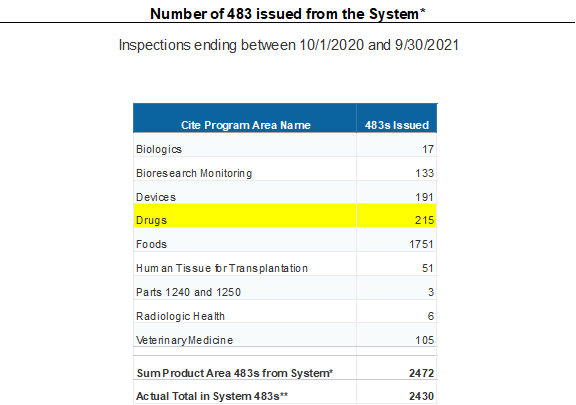
对于药品,该总结显示共发出215封FDA 483表格(FDA系统产生)。涉及235条的法规引用,其中对Part 211(成品制剂的cGMP要求)占了184条,即78%(2020年数据为79%),可以看出大部分观察项集中于GMP领域。
这235个的法规条款,共计被引用了1120次,其中前10大条款共计引用了360次,约占总引用次数的三分之一,这10个条款基本上代表药企在cGMP方面的主要问题,其中9个缺陷项出现在2020年的前10大缺陷中,另外一个新进入本年度第10大缺陷项的是员工培训不足的问题。
缺陷1:
质量部门相关的程序,21 CFR211.22(d)
2021年引用次数:80次(占7.1%)
2020年排名:同样是第1大的缺陷项,占总引用条款的7%
Procedures not in writing, fully followed
The responsibilities and procedures applicable to the quality control unit are not [in writing] [fully followed]. Specifically, ***
程序不是书面形式、未完全遵守
没有[书面] [完全遵守]适用于质量控制单位的责任和程序。具体来说,***
缺陷2:
调查不彻底,21 CFR211.192
2021年引用次数:49次(占4.4%)
2020年排名:同样是第2大的缺陷项,占总引用条款的5%
Investigations of discrepancies, failures
There is a failure to thoroughly review [any unexplained discrepancy] [thefailure of a batch or any of its components to meet any of its specifications]whether or not the batch has been already distributed. Specifically, ***
对差异、失效的调查
无法彻底检查[任何无法解释的差异] [批次或其任何组件未能满足其任何质量标准],无论批次是否已经分发。具体来说,***
缺陷3:
缺少书面程序,21 CFR211.100(a)
2021年引用次数:44次(占3.9%)
2020年排名:第4大的缺陷项,占总引用条款的3%
Absence of Written Procedures
There are no written procedures for production and process controlsdesigned to assure that the drug products have the identity, strength, quality,and purity they purport or are represented to possess. Specifically, ***
缺少书面程序
没有书面的生产和过程控制程序,可用来确保药品具有其声称或代表拥有的鉴别、强度、质量和纯度。具体来说,***
缺陷4:
实验室控制不充分,21 CFR211.160(b)
2021年引用次数:40次(占3.6%)
2020年排名:第3大的缺陷项,占总引用条款的4%
Scientifically sound laboratory controls
Laboratory controls do not include the establishment of scientificallysound and appropriate [specifications] [standards] [sampling plans] [testprocedures] designed to assure that [components] [drug product containers][closures] [in-process materials] [labeling] [drug products] conform toappropriate standards of identity, strength, quality and purity. Specifically, ***
科学合理的实验室控制
就实验室控制措施,未能包括建立科学合理且适当的[质量标准] [标准] [取样计划] [检验程序],以确保[组分] [药品容器] [密封件] [在制品] [标签] [药品]符合适当的鉴别、强度、质量和纯度标准。具体来说,***
缺陷5:
清洁问题,21 CFR211.67(a)
2021年引用次数:33次(占2.9%)
2020年排名:第6大的缺陷项,占总引用条款的3%
Cleaning / Sanitizing / Maintenance
Equipment and utensils are not [cleaned] [maintained] [sanitized] at appropriate intervals to prevent [malfunctions] [contamination] that would alter the safety, identity, strength, quality or purity of the drug product. Specifically, ***
清洁/消毒/维护
设备和器具未按适当的时间间隔进行[清洁] [维护] [消毒],以防止可能会改变药品安全性、鉴别、强度、质量或纯度的[故障] [污染]。具体来说,***
缺陷6:
计算机控制问题,21 CFR211.68(b)
2021年引用次数:30次(占2.7%)
2020年排名:第7大的缺陷项,占总引用条款的3%
Computer control of master formula records
Appropriate controls are not exercised over computers or related systems to assure that changes in master production and control records or other records are instituted only by authorized personnel. Specifically, ***
主记录的计算机控制
没有对计算机或相关系统进行适当的控制,以确保仅由授权人员来更改主生产和控制记录或其他记录。具体来说,***
缺陷7:
设备问题,21 CFR211.63
2021年引用次数:25次(占2.2%)
2020年排名:第5大的缺陷项,占总引用条款的3%
Equipment Design, Size and Location
Equipment used in the manufacture, processing, packing or holding of drug products is not [of appropriate design][of adequate size] [suitably located] to facilitate operations for its[intended use] [cleaning and maintenance]. Specifically, ***
设备设计、尺寸和位置
就生产、加工、包装或保存药品的设备,其没有[具有适当的设计] [没有足够的尺寸] [没有适当地放置],以实现其[预定用途] [清洁和维护]操作。具体来说,***
缺陷8:
无菌药品污染风险,21 CFR211.113(b)
2021年引用次数:22次(占2.0%)
2020年排名:同样是第8大的缺陷项,占总引用条款的2%
Procedures for sterile drug products
Procedures designed to prevent microbiological contamination of drug products purporting to be sterile are not [established] [written][followed]. Specifically, ***
无菌药品程序
没有[建立] [编写] [遵守]旨在防止药品无菌污染的设计程序。具体来说,***
缺陷9:
校验相关问题,21 CFR211.68(a)
2021年引用次数:19次(占1.7%)
2020年排名:第10大缺陷项,占总引用条款的2%
Calibration/Inspection/Checking not done
Routine [calibration] [inspection] [checking] of [automatic] [mechanical] [electronic]equipment is not performed according to a written program designed to assure proper performance. Specifically, ***
校验/检查/审查未完成
没有按照旨在确保适当性能的书面程序,对[自动] [机械] [电子]设备进行例行[校验] [检查] [审查]。具体来说,***
缺陷10:
员工培训不足,21 CFR 211.25(a)
2021年引用次数:18次(占1.6%)
2020年排名:第18大的缺陷项,占总引用条款的1%
Training--operations, GMPs, written procedures
Employees are not given training in [the particular operations they perform as part of their function] [current good manufacturing practices][written procedures required by current good manufacturing practiceregulations]. Specifically, ***
培训——操作、GMP、书面程序
员工未接受[作为其职能的一部分执行的特定操作][cGMP][ cGMP法规要求的书面程序]的培训。具体来说, ***
Ref.: [FDA][2020-11-24]2020 Inspection Observations
关于举办2026年度四川省药品生产企业质
各药品生产企业: 2026年是我国..四川省医药保化品质量管理协会召开第七
2025年12月17日,四川省医药保化品..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..