11月24日,美国FDA发布了2020财年检查观察项的汇总数据,对本财年483表格中引用的相关缺陷数据进行了总结。
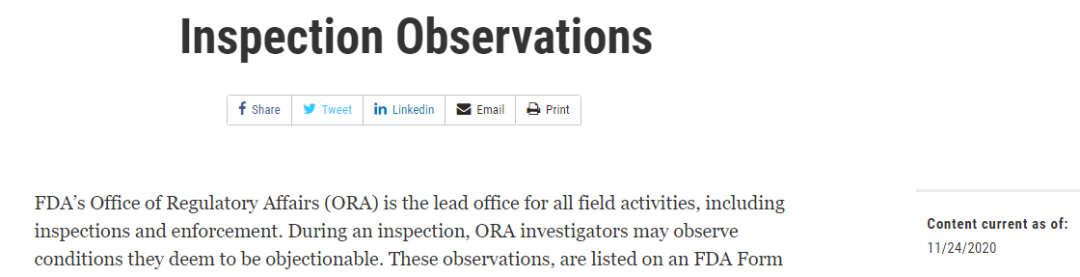
对于药品,该总结显示共涉及286条的法规引用,其中对Part 211(成品制剂的cGMP要求)占了226条,即79%,可以看出大部分观察项集中于GMP领域。
这286个的法规条款,共计被引用了1514次;其中被引用20次以上的有14条,合计引用次数为607次,占总引用次数的40%。这14个条款基本上代表药企在cGMP方面的主要问题:
条款1:21 CFR 211.22(d)
引用次数:111次(占7%)
Procedures not in writing, fully followed
The responsibilities and procedures applicable to the quality control unit are not [in writing] [fully followed]. Specifically, ***
程序不是书面形式、未完全遵守
没有[书面] [完全遵守]适用于质量控制单位的责任和程序。具体来说,***
条款2:21 CFR 211.192
引用次数:79次(占5%)
Investigations of discrepancies, failures
There is a failure to thoroughly review [any unexplained discrepancy] [the failure of a batch or any of its components to meet any of its specifications] whether or not the batch has been already distributed. Specifically, ***
对差异、失效的调查
无法彻底检查[任何无法解释的差异] [批次或其任何组件未能满足其任何质量标准],无论批次是否已经分发。具体来说,***
条款3:21 CFR 211.160(b)
引用次数:58次(占4%)
Scientifically sound laboratory controls
Laboratory controls do not include the establishment of scientifically sound and appropriate [specifications] [standards] [sampling plans] [test procedures] designed to assure that [components] [drug product containers] [closures] [in-process materials] [labeling] [drug products] conform to appropriate standards of identity, strength, quality and purity. Specifically, ***
科学合理的实验室控制
就实验室控制措施,未能包括建立科学合理且适当的[质量标准] [标准] [取样计划] [检验程序],以确保[组分] [药品容器] [密封件] [在制品] [标签] [药品]符合适当的鉴别、强度、质量和纯度标准。具体来说,***
条款4:21 CFR 211.100(a)
引用次数:46次(占3%)
Absence of Written Procedures
There are no written procedures for production and process controls designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. Specifically, ***
缺少书面程序
没有书面的生产和过程控制程序,可用来确保药品具有其声称或代表拥有的鉴别、强度、质量和纯度。具体来说,***
条款5:21 CFR 211.63
引用次数:44次(占3%)
Equipment Design, Size and Location
Equipment used in the manufacture, processing, packing or holding of drug products is not [of appropriate design] [of adequate size] [suitably located] to facilitate operations for its [intended use] [cleaning and maintenance]. Specifically, ***
设备设计、尺寸和位置
就生产、加工、包装或保存药品的设备,其没有[具有适当的设计] [没有足够的尺寸] [没有适当地放置],以实现其[预定用途] [清洁和维护]操作。具体来说,***
条款6:21 CFR 211.67(a)
引用次数:42次(占3%)
Cleaning / Sanitizing / Maintenance
Equipment and utensils are not [cleaned] [maintained] [sanitized] at appropriate intervals to prevent [malfunctions] [contamination] that would alter the safety, identity, strength, quality or purity of the drug product. Specifically, ***
清洁/消毒/维护
设备和器具未按适当的时间间隔进行[清洁] [维护] [消毒],以防止可能会改变药品安全性、鉴别、强度、质量或纯度的[故障] [污染]。具体来说,***
条款7:21 CFR 211.68(b)
引用次数:38次(占3%)
Computer control of master formula records
Appropriate controls are not exercised over computers or related systems to assure that changes in master production and control records or other records are instituted only by authorized personnel. Specifically, ***
主记录的计算机控制
没有对计算机或相关系统进行适当的控制,以确保仅由授权人员来更改主生产和控制记录或其他记录。具体来说,***
条款8:21 CFR 211.113(b)
引用次数:31次(占2%)
Procedures for sterile drug products
Procedures designed to prevent microbiological contamination of drug products purporting to be sterile are not [established] [written] [followed]. Specifically, ***
无菌药品程序
没有[建立] [编写] [遵守]旨在防止药品无菌污染的设计程序。具体来说,***
条款9:21 CFR 211.160(a)
引用次数:31次(占2%)
Following/documenting laboratory controls
Established [specifications] [standards] [sampling plans] [test procedures] [laboratory control mechanisms] are not [followed] [documented at the time of performance]. Specifically, ***
遵守/记录实验室控制
对于既定的[质量标准] [标准] [取样计划] [检验程序] [实验室控制机制],其未[遵守] [执行时有书面记录]。具体来说,***
条款10:21 CFR 211.68(a)
引用次数:29次(占2%)
Calibration/Inspection/Checking not done
Routine [calibration] [inspection] [checking] of [automatic] [mechanical] [electronic] equipment is not performed according to a written program designed to assure proper performance. Specifically, ***
校验/检查/审查未完成
没有按照旨在确保适当性能的书面程序,对[自动] [机械] [电子]设备进行例行[校验] [检查] [审查]。具体来说,***
条款11:21 CFR 211.192
引用次数:28次(占2%)
Written record of investigation incomplete
Written records of investigations into [unexplained discrepancies] [the failure of a batch or any of its components to meet specifications] do not [always] include the conclusions and follow-up. Specifically, ***
书面调查记录不完整
就[无法解释的差异] [批次或其任何组件未达到质量标准要求]进行的调查,其书面记录[并不总是]包括结论和后续行动。具体来说,***
条款12:21 CFR 211.67(b)
引用次数:26次(占2%)
Written procedures not established/followed
"Written procedures are not [established] [followed] for the cleaning and maintenance of equipment, including utensils, used in the manufacture, processing, packing or holding of a drug product. Specifically, ***
未建立/遵守书面程序
“没有[建立] [遵守]书面程序,来清洁和维护用于生产、加工、包装或保存药品的设备,包括小器具。特别是,***
条款13:21 CFR 211.165(a)
引用次数:22次(占1%)
Testing and release for distribution
Testing and release of drug product for distribution do not include appropriate laboratory determination of satisfactory conformance to the [final specifications] [identity and strength of each active ingredient] prior to release. Specifically, ***
检验和放行销售
检验和放行要分发的药品时,在放行前,未能对[最终质量标准] [每种活性成分的鉴别和强度]进行充分适当的实验室确认。具体来说,***
条款14:21 CFR 211.110(a)
引用次数:22次(占1%)
Control procedures to monitor and validate performance
"Control procedures are not established which [monitor the output] [validate the performance] of those manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product. Specifically, ***
相关控制程序,以监控和验证性能
“没有建立控制程序,就那些可能导致过程中材料和药品特性变化的生产工艺,进行[监控输出] [验证性能]。具体来说,***
Ref.: [FDA][2020-11-24]2020 Inspection Observations
关于举办2026年度四川省药品生产企业质
各药品生产企业: 2026年是我国..四川省医药保化品质量管理协会召开第七
2025年12月17日,四川省医药保化品..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..